
$84.00
This manual is appropriate for courses populated primarily by allied health students or for courses where an abbreviated number of experiments is preferred. This new edition has been carefully revised to provide increased clarity, better organization, and improvements to its already unsurpassed photography and artwork. These features combine to make Microbiology: Laboratory Theory & Application the best-selling microbiology lab manual series on the market.
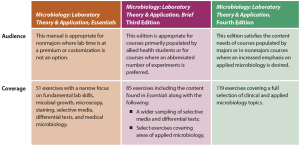
The following grid illustrates the primary audience and coverage differences between the three titles in the Microbiology: Laboratory Theory and Application series.
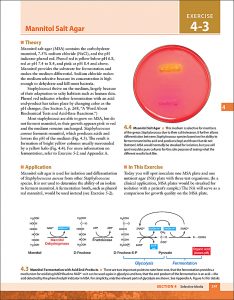
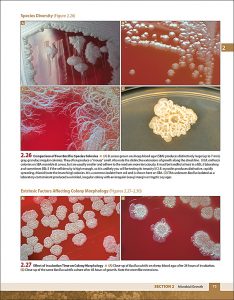
Examples of interior pages:

Instructor Resources Available: Login to view Instructor Resources
A version of this manual is available with microbiology lab kits created by Avantor/VWR (Ward's Science): link. The manual aligns with the distance learning lab exercises available within the kit.